Abstract
Oral ruxolitinib has been successfully used for the treatment of acute and chronic graft-versus-host disease (cGvHD) and topical ruxolitinib has demonstrated efficacy in clinical trials for vitiligo, atopic dermatitis, and psoriasis.
Background: There are no FDA-approved topical treatments for cutaneous cGvHD. Topical corticosteroids are the mainstay of skin-directed therapy for the inflammatory-phase cutaneous cGvHD but are associated with significant side effects such as skin thinning, bruising, striae, infections, and acne, and may incompletely treat cutaneous cGvHD, prompting use of systemic therapies.
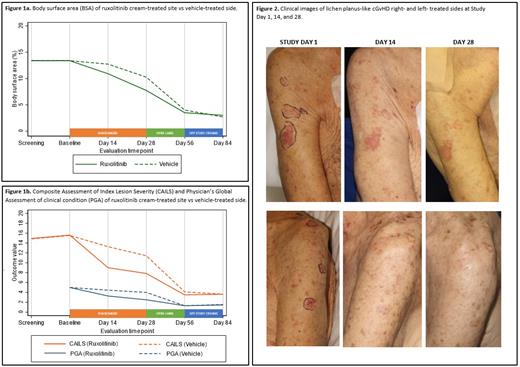
Methods. We conducted a prospective, randomized, double-blind, vehicle- and comparator-controlled, phase 2, proof-of-concept trial evaluating the efficacy and safety of topical ruxolitinib 1.5% cream in patients 12 years of age or older with cutaneous nonsclerotic (lichen-planus like, poikilodermatous) and superficially sclerotic (lichen sclerosus, morphea-like) cGvHD with ≥2% of body surface area (BSA) affected at a single, academic transplantation center in the United States. Patients were only eligible to enroll if systemic therapy, when applicable, was stable for ≥ 4 weeks and concurrent topical therapy (including phototherapy) was not used. Patients were randomly assigned (1:1) to receive topical ruxolitinib 1.5% cream left side of face/body or right side of face/body with placebo vehicle cream to contralateral side of face/body twice daily to for 28 days, followed by an optional open label extension to both sides of 28 days for interested patients. The primary end point was efficacy as measured by BSA of the GvHD rash on the side of face/body treated with topical ruxolitinib cream vs contralateral side treated with vehicle at Day 28. Secondary endpoints were Physician's Global Assessment of clinical condition (PGA) and Composite Assessment of Index Lesion Severity (CAILS) of the ruxolitinib-treated side vs vehicle-treated side at Day 14 and 28. Interim analyses were performed once 10 patients were evaluable of the planned 24 patients.
Results. Between 6/28/19 and 5/14/21, a total of 13 patients (mean age 52.6 years [SD 20.0]; 7 [54%] female and 6 [46%] male) underwent randomization; 11 patients completed Day 14 assessments, 12 patients Day 28 assessments, and 10 patients Day 56 assessments. Patients had a history of acute leukemia (N=8 [62%]), non-Hodgkin lymphoma (N=3 [23%]), myeloproliferative neoplasm (N=1 [8%]), or aplastic anemia (N=1 [8%]). Median time from transplant to enrollment was 665 days (IQR 433-1355), and from cGvHD onset to enrollment 283 days (IQR 115-867). Chronic GvHD was NIH mild (8%), moderate (23%), or severe (62%), predominantly classic (85% vs. overlap 15%), with 46% having 4 or more involved organs. Most patients were enrolled for treatment of cutaneous nonsclerotic cGvHD (N=10, 77%) with lichen planus-like (N=8), papulosquamous (N=1), and maculopapular rash/erythema features (N=1). Three patients were enrolled for treatment of lichen sclerosus-like cGvHD. Patients were heavily pretreated with 4 (31%) having 3 or more prior lines of systemic therapy for cGvHD. Most patients had failed at least 2 topical therapies, with 77% previously failed topical steroids, 24% topical calcineurin inhibitors, and 24% phototherapy. There was a trend in reduced BSA of cGvHD on the treatment side compared to the vehicle side from Day 1 (13.4 on treatment/vehicle) to Day 14 (10.9 vs 13.8; p=0.06) and continuing to Day 28 (7.7 vs 11.0; p=0.15), respectively. PGA (Day 1: 5 treatment/vehicle) of treatment side was significantly improved starting at Day 14 (3.3 treatment vs 4.4 vehicle; p=0.024), with continued improvement at Day 28 (2.5 vs 4.0; p= 0.026). CAILS (Day 1: 15.6 treatment vs 15.5 vehicle; p=0.83) of treatment side was also significantly improved starting at Day 14 (9.0 vs 13.3; p=0.02). There were no serious adverse events (SAEs) reported. One patient had a grade 1 headache which was attributed possibly to therapy. Three patients had treatment-emergent AEs (all grade 1) that were unlikely (N=1) or unrelated (N=2) to study therapy.
Conclusions. Topical ruxolitinib 1.5% cream was effective in treating cutaneous nonsclerotic and superficially sclerotic GvHD as determined by PGA and CAILS. These data suggest that ruxolitinib cream might be a safe and effective treatment option for patients with cutaneous nonsclerotic and superficially sclerotic chronic GvHD.
Markova: Alira Health Ventures: Consultancy; Incyte Corporation: Research Funding; Blueprint Medicines: Consultancy; UpToDate: Patents & Royalties: Royalties for chapter on dermatologic adverse events to targeted therapies ; Amryt Pharma: Research Funding. Perales: MorphoSys: Honoraria; Omeros: Honoraria; Cidara: Honoraria; Incyte: Honoraria, Other; Equilium: Honoraria; Medigene: Honoraria; Kite/Gilead: Honoraria, Other; Karyopharm: Honoraria; Sellas Life Sciences: Honoraria; Novartis: Honoraria, Other; Nektar Therapeutics: Honoraria, Other; Servier: Honoraria; Miltenyi Biotec: Honoraria, Other; NexImmune: Honoraria; Celgene: Honoraria; Merck: Honoraria; Takeda: Honoraria; Bristol-Myers Squibb: Honoraria. Prockop: Memorial Sloan Kettering Cancer Center: Other: S Prockop receives support for the conduct of sponsored clinical trials through MSK from Atara Biotherapeutics, Jasper and AlloVir. , Patents & Royalties: S Prockop is a co-inventor on intellectual property (IP) licensed to Atara. S Prockop has waived rights to this IP to MSK and has no personal financial interests in Atara. MSK has financial interests in Atara and IP interests relevant to this abstract. ; MSK: Other: Inventor; Neovii: Consultancy; ADMA Biologics: Consultancy; Jasper: Other: support for the conduct of sponsored trials; AlloVir: Other: support for the conduct of sponsored trials; Atara Biotherapeutics: Other: support for the conduct of sponsored trials and Inventor. Ponce: Kadmon pharmaceuticals: Consultancy, Honoraria; Ceramedix: Consultancy, Honoraria; CareDx: Consultancy, Honoraria; Seres Therapeutics: Consultancy, Research Funding; Generon Pharmaceuticals: Consultancy; Takeda Pharmaceuticals: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal